Potential Changes to the Platelet Ecosystem
/contact: bw@bloodbuy.com
Radical change is coming to the hospital-blood center relationship. The cause will be a new guidance[i] from the FDA requiring re-testing of platelets after 3 days of storage. The guidance is currently a draft, but industry insiders expect its release in final form this spring or summer.
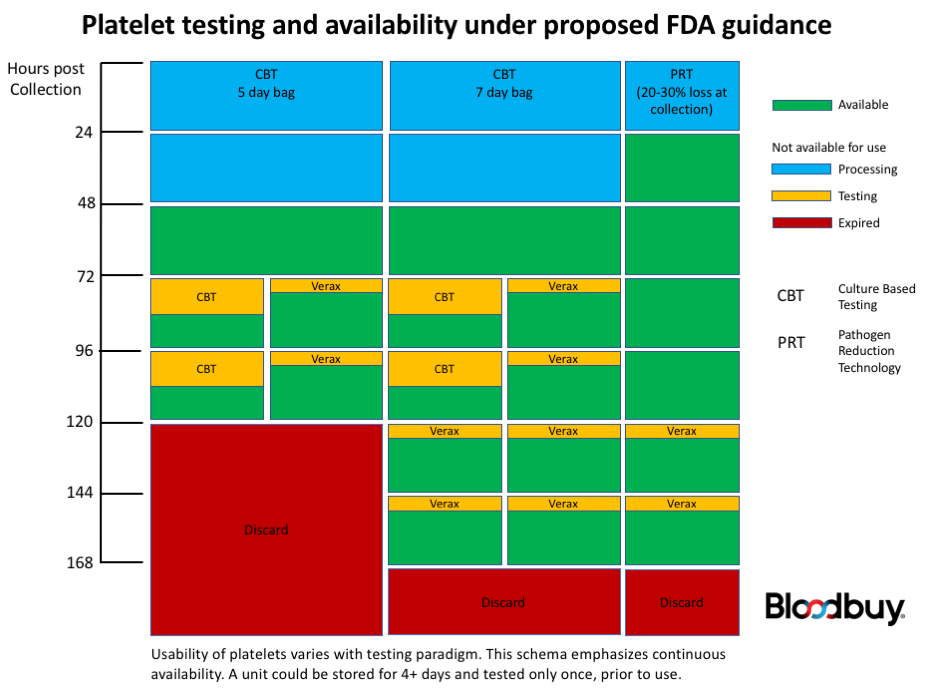
Most platelets will require additional testing after 3 days under the proposed guidance
New guidance always creates a certain amount of disruption as blood centers adapt to new workflow, and as additional incremental costs make their way into the system. The most recent guidance[ii], for example, required testing for ZIKV in all collections. As a new test, it added a marginal cost and one additional test before products could be released for use.
The paradigm shift with the newest guidance lies in its disruption of the common-place “consignment model” of platelet distribution, as well as the requirement that that transfusion services, housed within hospitals, may be required to run these new tests on platelets.
In the current model, blood centers handle most testing prior to release of blood products to transfusion centers. Additionally, transfusion centers often retain relatively small stocks on hand to minimize storage overhead and wastage of unused stocks. Before the product expiration date, unused stock can be rotated back to the blood center for redistribution of the short-dated product to hospitals with an immediate need.
Presently, the draft guidance would require that platelets either be treated with pathogen reduction technology (PRT) after collection, or be subject to a culture-based test. On the 4th and following days post-collection, non-PRT platelets would require an additional test for bacterial contamination using either a culture-based method or a rapid immune-assay-based test. Critically, units re-tested on day 4 would require an additional bacterial contamination test if used more than 24 hours following the first re-test.
One benefit from the guidance is the potential to extend the usable life of platelets to 7 days post-collection, provided they are in 7-day approved bags. Both PRT units and units subject to initial culture-based testing could be extended to 7 days, although additional rapid tests would be required for each 24-hour extension beyond 5 days. There are currently no culture-based tests that could be used to extend platelet usability beyond 5 days post-collection.
Although PRT would eliminate the requirement for re-testing on days 4 or 5, there are drawbacks. Under currently approved protocols the only PRT approved for use in the US, Cereus’ Intercept[iii], results in significant loss of single-donor platelet collections (SDP). Experienced operators report a loss of 15-20% of a single collection. Pooled platelets and those derived from whole blood collections are not eligible for PRT under currently approved rules. With platelet stocks already strained, such a reduction in collection volume could result in significant nationwide shortages. Additionally, the PRT process increases the unit cost of platelets by 25-30%.
The core issue is that non-PRT platelets, under the draft guidance, would be viable for only 3 days post collection before additional testing for bacterial contamination would be required. Given current manufacturing protocols, that equates to 1 day of shelf life after delivery to a transfusion center. Viability can be extended beyond that three-day window, but repeated testing carries significant logistic, staff, and monetary costs.
In conversations with multiple industry experts, the prevailing opinion is that the only approved rapid test, Verax PGD from Fenwal[iv], is not trivial to perform. The protocol uses very small volumes, is not automated, and requires some skill to administer properly. One 12-year industry veteran believes that a medical technologist (MT) would be required to adequately perform the tests; compounding a pre-existing shortage of MTs and the staffing issues many facilities are currently juggling. It is worth noting that opinion on this subject varies, although even those commentators[v] who do not find Verax testing cumbersome do have reservations regarding how products are labeled and classified pre- and post-treatment.
One bright spot in the new guidance is that platelets collected in bags approved for extended storage can be used up to 7 days post-collection, provided they undergo a rapid test no more than 24 hours prior to transfusion. The simpler and more familiar culture-based testing can be used to extend viability only up to 5 days post collection. Note, however, that a minimum of 12 hours of incubation is commonly required to perform culture-based testing. Under optimal conditions, that leaves only 12 hours to transfuse a unit of platelets after secondary testing with a growth-based assay.
Once re-tested, each platelet bag needs either a label modification or an attached tag to indicate the method of testing and the revised expiration date and time. Although that requirement may appear trivial, many facilities will not be equipped to produce compliant labeling. Instituting this measure will be in addition to the workload involved in the actual testing.
Under the existing paradigm, where nearly all safety testing is carried out by blood centers, very few hospitals are prepared for the proposed platelet-specific testing regimen. In the modern era of out-sourcing reference and microbiology testing labs, many facilities will simply be unable to complete the tests on-premises. Establishing a protocol for sampling, testing, and tracking results will be a significant logistical, procedural, and IT challenge.
The draft guidance does explicitly permit blood products to be returned to the blood center for additional testing, potentially relieving the transfusion center of having to conduct the tests in-house. The logistics, however, are daunting. With a 24-hour window between test and use the turnaround would have to be very tight, indeed. If a culture-based test is used instead of Verax, only 12 hours separate the conclusion of the test from the expiration of the new period of viability. With many transfusion centers now sourcing blood products from distributed networks, a 24-hour turnaround would simply be impossible. The consignment model also becomes untenable as blood centers simply cannot assume the inventory risk burden of multiple tests for each unit of platelets.
The Rationale
The impetus for the guidance is concern over bacterial sepsis resulting from platelet transfusion. According to the FDA guidance documentation, there were 13 deaths recorded due to transfusion-associated sepsis (TAS) from 2009 to 2013. As Hong et al[vi] point out, however, there may be significant under-reporting of adverse reactions due to exposure to bacteria in platelet products. The incidence may be as high as 1 in 2,000 transfusions, and many adverse reactions may be hidden by the already precarious health of many recipients and the long lag time between the establishment of a cryptic infection and detectable impact on health. The concern with TAS has reached both industry journals[vii],[viii] and public media[ix].
Platelets are a particular concern for bacterial exposure because they are stored at room temperature, either in plasma or a nutrient solution that provides an excellent growth environment for many potential pathogens. Initial contamination with even a few bacteria can, thanks to exponential growth, lead to levels that can cause illness or death. Other blood products are frozen or stored at 2-4 deg C, preventing rapid post-collection growth.
The current system of post-collection culture-based screening has been very successful at reducing the incidence of TAS (statistics). As Hong et al note, however, culture-based screening can overlook low initial contamination and slow-growing organisms. Of the 13 TAS deaths, all had initially tested negative using culture-based screens. All of the deaths occurred from units on day 4 or 5 of viability[i], indicating that growth of an initially low-level contamination was at fault.
The stated goal of the FDA’s draft guidance is to further reduce exposure to bacterial contamination by either inactivating pathogens present at currently-undetectable levels using PRT, or to prevent transfusion of products that have experienced growth of bacteria subsequent to the initial test.
Resolutions to an Impasse
Although well-intentioned, the proposed guidance will place a significant burden both on blood centers, and on transfusion centers that are not equipped to perform point-of-use testing. The net effect could be a further reduction in platelet availability even as the donor pool is declining, and additional deferrals for TRALI-associated risks are being proposed.
Pre-storage pooled platelets (AcrodoseTM) derived from whole blood donation (WBD) have been approved by the FDA since 2005, and could represent an important additional resource. Although pooled platelets are widely used in Europe, adoption has been slow in the United States. Additionally, the FDA has not approved PRT for use with WBD platelets, and they are not eligible for 7-day storage. Finally, they must undergo the same testing regimen as SDPs (Verax or CBT if transfused more than 3 days post manufacture and within 24 hours prior to transfusion).
Several potential alternatives to the proposed guidelines have been suggested in comments submitted for the guidance[x]. One set of suggestions focus on altering how PRT is used[xi],[xii]. If the guard bands on Intercept could be made more permissive such that a higher percentage of platelet donations could be retained. Also, as the comment from Cereus indicates, PRT-treated platelets have shown no indication that bacterial contamination is not an issue post treatment. If PRT platelets are “functionally sterile”, as stated in the comment, follow-up testing would not be necessary even through day 7. The longer period of viability, increasing the efficiency of the collection process, and elimination of secondary bacterial testing would make PRT a much more attractive option.
The second set of suggestions focus on changing guidelines for the initial culture-based screen for non-PRT units [xi],[xiii]. Large initial sample size coupled with extended incubation could potentially detect more false-negative units. The FDA guidance and comments from blood centers cite sources that disagree on the efficacy of this approach. If a consensus could be reached, however, more rigorous initial testing could substitute for the follow-on testing. The extended-culture screen would have some logistical challenges, such as the challenge of incubating media for an extended period for all units, as well as potentially having to notify recipients of bacterial exposure post-transfusion. Many blood centers already follow such a protocol, however.
Critically, both of the suggested approaches keep the burden of increased testing on the blood centers. Some consideration would have to be given to the additional cost that would be borne by the collection facilities, but such a system would maintain the core centers of excellence as-is.
There has also been a call to reopen the guidance to commentary as many transfusion services were previously unaware of the impact this guidance would impose. Indeed, most of the comments in the docket are from blood centers, rather than hospitals that would potentially need to adapt to a shortage of platelets and/or a changed testing regimen. If the guidance is published as-is, however, transfusion services and blood centers will have just 12 months to re-engineer already precarious platelet logistics. Discussions surrounding how this guidance will be implemented need to begin now.
Sources
[i] Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion. (2016, March). Retrieved April 21, 2017, from https://www.fda.gov/downloads/Guidances/Blood/UCM425952.pdf
[ii] Revised Recommendations for Reducing the Risk of Zika Virus Transmission by Blood and Blood Components. (2016, August). Retrieved April 21, 2017, from https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/blood/ucm518213.pdf
[iii] https://intercept-usa.com/
[iv] http://veraxbiomedical.com/products/platelet-pgd-test.asp
[v] https://www.regulations.gov/document?D=FDA-2014-D-1814-0115
[vi] Hong, H., & Xiao, W. (2016). Detection of septic transfusion reactions to platelet transfusions by active and passive surveillance. Blood,127(4), 496-502. doi:https://doi.org/10.1182/blood-2015-07-655944
[vii] Blood Safety. (2013, March 21). Retrieved April 21, 2017, from http://www.cdc.gov/bloodsafety/bbp/bacterial-contamination-of-platelets.html
[viii] Aubron, C., Flint, A. W., Bailey, M., Pilcher, D., Cheng, A. C., Hegarty, C., . . . McQuilten, Z. (2017, January 06). Is platelet transfusion associated with hospital-acquired infections in critically ill patients? Retrieved April 21, 2017, from http://ccforum.biomedcentral.com/articles/10.1186/s13054-016-1593-x
[ix] Osborn, J. (2015, March 17). After Years Of Delay, Will The FDA Finally Make Safe Our Nation's Blood Supply? Retrieved April 21, 2017, from http://www.forbes.com/sites/johnosborn/2015/03/17/after-years-of-delay-will-the-fda-finally-make-safe-our-nations-blood-supply/#39660b4b6950
[x]https://www.regulations.gov/docketBrowser?rpp=25&so=DESC&sb=postedDate&po=0&D=FDA-2014-D-1814
[xi] America’s Blood Centers: https://www.regulations.gov/document?D=FDA-2014-D-1814-0117
[xii] Cerus: https://www.regulations.gov/document?D=FDA-2014-D-1814-0097
[xiii] New York Blood Center: https://www.regulations.gov/document?D=FDA-2014-D-1814-0026